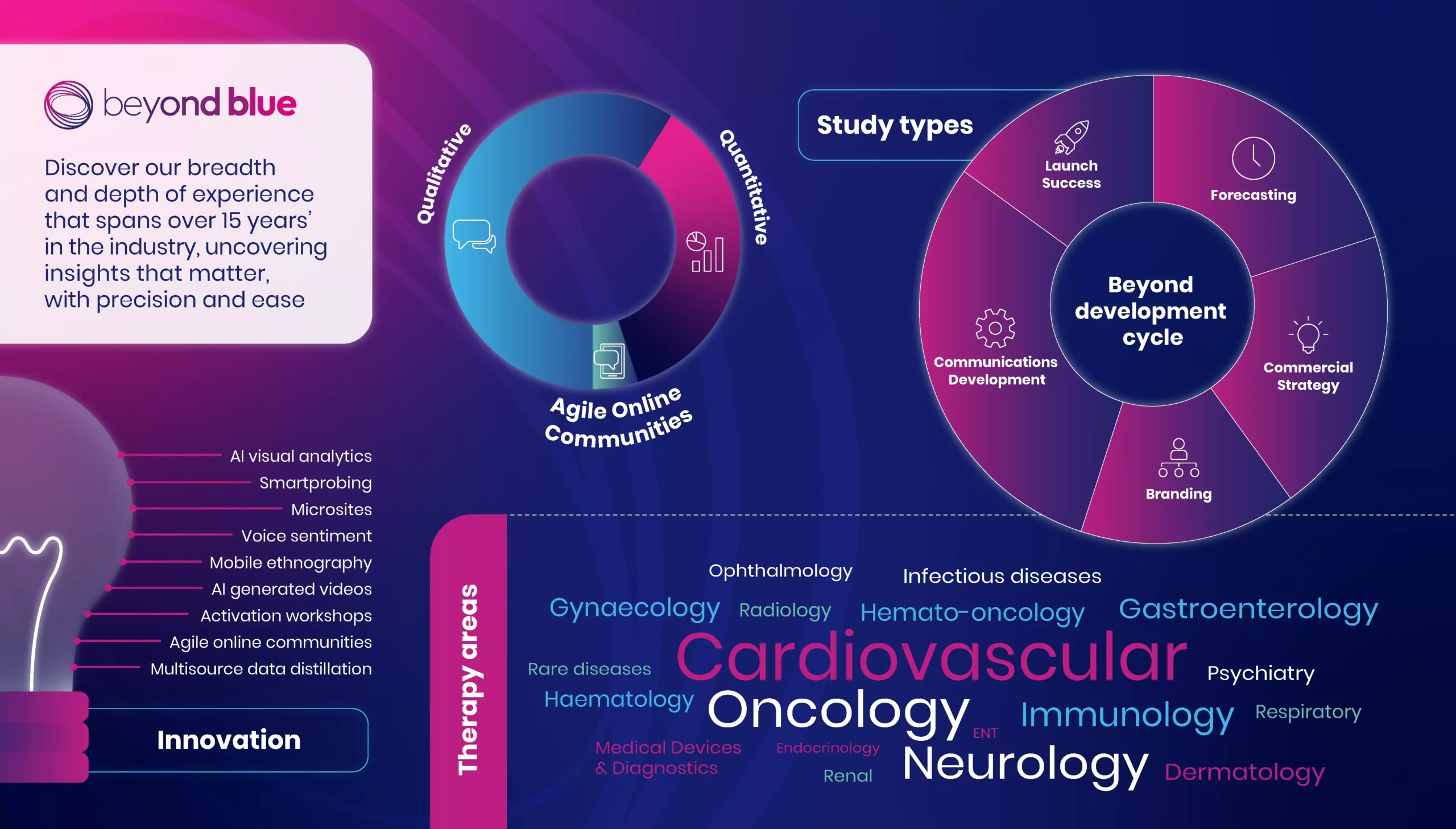
Why Beyond Blue
Achieve your pharmaceutical goals with clarity and precision.
Let Beyond Blue provide the clarity and precision you need to focus on what truly matters.
Navigate the intricate landscape of pharmaceutical market research seamlessly and receive prompt and dedicated support, accompanied by action-ready insights.
Let us help you steer through the complexity and remain focused on achieving your pharmaceutical business goals with confidence and clarity.
Our specialties:
Patient journey mapping and buying processes
Early opportunity assessment
Forecasting
Segmentation
Positioning and communications
Our directors are our core researchers. You’ll receive senior-level director involvement throughout your project, with expertise, guidance and commercial recommendations at every stage.
You’ll be supported by a strong structure of technically astute moderators in each market, along with an in-house team of 48+ project managers and data analysts.
We have worked with the majority of the top global pharma companies and our research experience covers all major markets across the globe including Western and Eastern Europe, US, Canada, Asia and Latin America.
Beyond
deliverables
Transform your insights into impactful actions: our mission is to ensure that your organization not only receives valuable insights but fully integrates them to drive success.
Get engaging and inspiring reports that are also actionable. We prioritize proactive conversations about data application, ensuring that we identify and engage with key stakeholders effectively.
We understand audiences can be weary of ‘death by PowerPoint’, which is why we go above and beyond in our presentations and feedback to make sure we share insight that isn’t only creative and interactive, but is also impactful and memorable. We do this by using animation, infographics, and visually immersive experiences to bring projects to life.
Case studies
Forecasting
Revolutionizing Gene Therapy with Strategic Conjoint Insights
Deep dive oncology
Supporting complex launches with our agile Wisdom of Crowds approach
Strategic Workshop
CardioCraft: Crafting a Strategic Brand House from Market Insights for Optimal Positioning in Cardiology
Learn more
Learn more
Learn more
Case studies
Forecasting
Revolutionizing Gene Therapy with Strategic Conjoint Insights
Learn more
Deep Dive Oncology
Supporting complex launches with our agile Wisdom of Crowds approach
Learn more
Strategic Workshop
CardioCraft: Crafting a Strategic Brand House from Market Insights for Optimal Positioning in Cardiology
Learn more
Compliance
We understand the importance of compliance and ensuring only the highest quality standards are adhered to.
At Beyond Blue, each member of our team is regularly trained and is compliant with the appropriate guidelines and processes (from ethics to pharmacovigilance), equipping them to deliver local standards and expectations across the UK, US, Europe, and beyond.
We are registered with the Information Commissioner’s Office (ICO), the UK’s independent authority set up to uphold information rights and data protection in the public interest. We adhere to principles of good practice as outlined by:
- ABPI (The Association of the British Pharmaceutical Industry)
- BHBIA (British Healthcare Business Intelligence Association)
- MRS (Market Research Society)
- EPHMRA (European Pharmaceutical Market Research Association)
- ESOMAR (European Society for Opinion and Marketing Research)
- Insights Association
Compliance Manager, Rhiannon Notman, is responsible for our business’s alignment with these principles and is a member of the ‘EPHMRA Ethics and Compliance committee’, helping us maintain an industry-leading standard and best practice for our clients.
They also oversee quality, compliance, and adverse event reporting across all of our projects and client accounts, including the implementation and monitoring of GDPR standards.
Our Research Director, Mike Pepp, is an active agency member of the EPHRMA Board, regularly attending and speaking at conferences and convening bespoke sessions.
Compliance
We understand the importance of compliance and ensuring only the highest quality standards are adhered to.
At Beyond Blue, each member of our team is regularly trained and is compliant with the appropriate guidelines and processes (from ethics to pharmacovigilance), equipping them to deliver local standards and expectations across the UK, US, Europe, and beyond.
We are registered with the Information Commissioner’s Office (ICO), the UK’s independent authority set up to uphold information rights and data protection in the public interest. We adhere to principles of good practice as outlined by:
ABPI (The Association of the British Pharmaceutical Industry)
BHBIA (British Healthcare Business Intelligence Association)
MRS (Market Research Society)
EphMRA (European Pharmaceutical Market Research Association)
Compliance Manager, Rhiannon Notman, is responsible for our business’s alignment with these principles and is a member of the ‘EPHMRA Ethics and Compliance committee’, helping us maintain an industry-leading standard and best practice for our clients.
They also oversee quality, compliance, and adverse event reporting across all of our projects and client accounts, including the implementation and monitoring of GDPR standards.
Our Research Director, Mike Pepp, is an active agency member of the EPHRMA Board, regularly attending and speaking at conferences and convening bespoke sessions.